Basics of Gas Chromatography – Mass Spectrometry
In order to understand Gas Chromatography-Mass Spectrometry (GCMS), it is best to start with a basic diagram of the device. Figure 1 below shows that the device is divided into three major parts. The first part is the gas chromatograph. The second part is the mass spectrometer. The third is the computer.
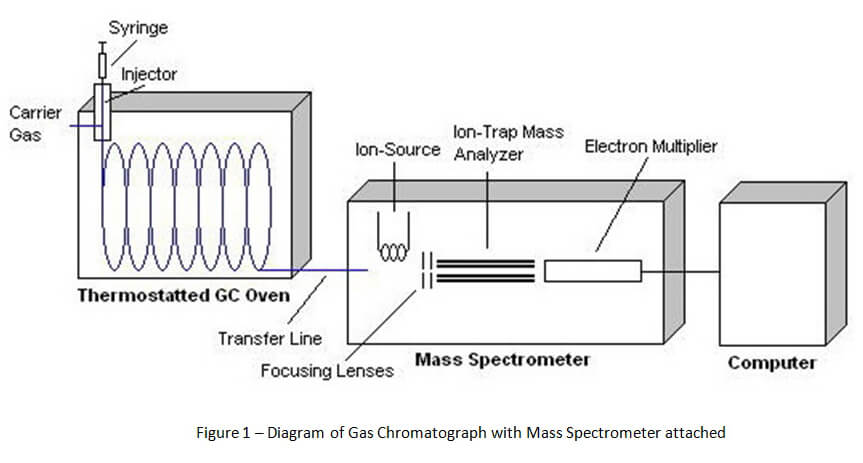
Unlike headspace gas chromatography that is used for ethanol detection, the method of gas chromatography for solid drug identification starts with direct injection. A vial of the suspected drug sample is prepared by the chemist for direct injection into the gas chromatograph by an auto-sampler. [1] This sample vial is placed onto a carousel with a number of other samples.
The auto-sampler inserts a syringe through the vial septum into the drug sample. A portion of the sample is extracted and then injected into the injector port for the gas chromatograph (See Figure 2 below). The sample is instantly vaporized by heat inside the injector port and sent into the column for the gas chromatograph via carrier gas.
A column (capillary column) is a long piece of coiled, hollowed wire that is lined by chemically coated glass. (See Figure 3 below). The chemical coating (stationary phase) inside the column and high temperature inside the gas chromatograph’s oven separates chemical compounds in the drug sample as it travels through the column. Ideally, the process of separation causes different chemical compounds to leave the column at separate times for proper identification. As the carrier gas (typically helium) carries the sample through the column, certain compounds tend to stick to the stationary phase for specific periods of time. Because the carrier gas is continually moving through the heated column, these chemical compounds can only stick to the stationary phase for a short period of time before they are carried out of the column and into the detector attached to the end of the gas chromatograph.

There are a number of different types of detectors that can be attached to gas chromatographs. For ethanol detection, the most common detector is a flame ionization detector (FID). However, the FID is not an appropriate detector for identifying solid drugs. Commonly, a mass spectrometer is used for qualitative and quantitative analysis for drugs of abuse.
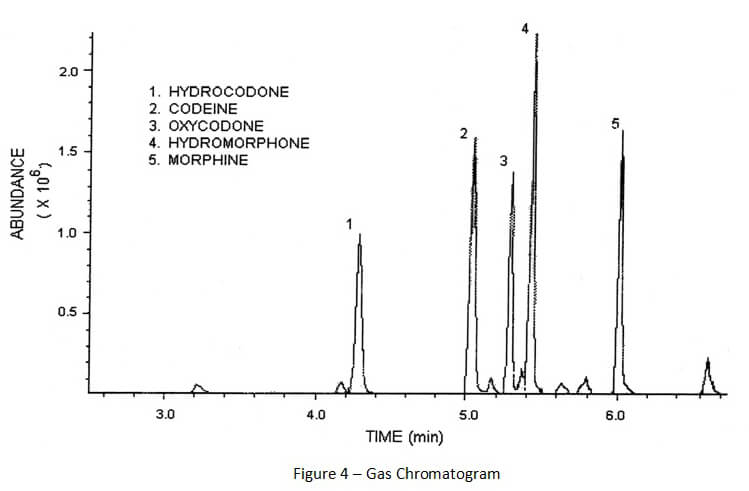
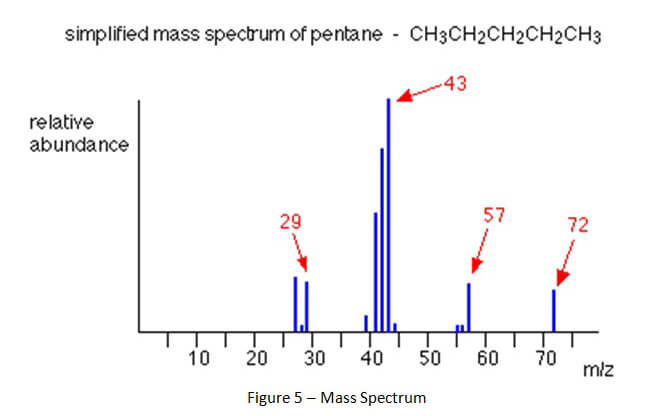
As compounds are separated and leave the gas chromatograph column, those compounds are destroyed by the attached detector. This process of destruction creates signals that are detected and then interpreted by a computer attached to the detector. A computer program interprets the signals and creates a gas chromatogram that is associated with the gas chromatography and creates a mass spectrum that is associated with mass spectrometry. (See Figures 4 and 5 below).
A gas chromatogram shows when chemical compounds left the column (eluted). When proper chromatography is achieved, there should be space between the peaks representing each chemical compound. Furthermore, those peaks should be skinny and symmetrical.
A mass spectrum shows something entirely different than the gas chromatogram. When a chemical compound goes through the entrance of the mass spectrometer, it is blasted into all of its component parts. The separate parts are called ions. The ions then continue through the mass spectrometer until they hit the detector. The ions have unique molecular weights which can be represented in a mass spectrum like Figure 5, below.
The computer program will then compare the different types of ions that are present to a library of spectrums. The computer program lists the compounds contained in the library that
are most like the compound that went through the mass spectrometer. This is called a “hit list.” Think of a bicycle being taken apart by one person and then a second person sifts through the parts to figure out what those parts can be used to build. The second person sees two wheels, handle bars, a bell, pedals, a frame, a roller chain, and a seat. That person then makes an educated guess that these parts made up a bicycle before it was disassembled.
The hit list may have numerous different compounds that are similar to the sample compound. Each compound on the list has a percentage assigned to it called a “match factor.” A sample compound identified by the computer program may have a low match factor. In other words, the match factor could be as low as 40% or 30%.
A chemist who is doing his or her job properly needs to review the gas chromatograms and mass spectrum, in addition to the computer generated matches, in order to give a proper opinion about the identification of the sample compound. A chemist should never base an opinion solely upon which compound match is at the top of the hit list. The gas chromatogram must show the sample compound left (eluted) the gas chromatograph at a particular time for it to be identified as a particular compound. Additionally, the mass spectrum must show particular types of ions to be present in order to make a proper identification of the sample compound.
[1] The importance of proper sample preparation will be explored in a subsequent article.

